malaria vaccine trials
The results were published on February 23 2017 in Nature. More than 400000 people a year die.

Malaria Vaccine Candidates In Clinical Development Data Sources For Download Scientific Diagram
For successful vaccine integration into malaria intervention programs the relational dynamics between researchers and trial communities must be made explicit.

. In December 2016 an expert meeting was convened at NIAID NIH in Rockville Maryland to deliberate on the rationale and design of malaria vaccine trials in pregnant women. The approach is now being tested in a Phase 2 clinical trial in Mali. Phase 1 clinical trials of a novel candidate malaria vaccine have found that the regimen conferred unprecedentedly high levels of durable protection when volunteers were.
A large scale malaria vaccine study led by the world health organization has been criticised by a leading bioethicist for committing a serious breach of international ethical standards. A Phase 2 clinical trial of the vaccine is now underway in Mali a malaria-endemic country. Another pre-erythrocytic vaccine candidate R21 recently showed good efficacy in an early trial testing it among children 5 17 months of age in Burkina Faso.
Clinical trials are a critical source of data for decisions around the development of vaccines drugs and other medical interventions. Two VAR2CSA-based vaccines PAMVAC and PRIMVAC aimed at inhibiting placental malaria have recently completed phase 1 clinical trials and were immunogenic and had acceptable safety profiles but. The Centers for Disease Control and Prevention welcomes the announcement today that results from the clinical trial in Africa of a malaria vaccine candidate show it prevented about half of malaria cases including the most severe in young children.
They are carried out in several phases. Vaccine introduction is by the respective MoH in selected areas randomly assigned to receive the vaccine at. Phase 3 trial results for vaccine RTSSAS01 7 April 2017 QA.
Ally Olotu on malaria vaccine research. In a trial in 450 children aged 517 months the vaccine called R21 was up to 77 effective at preventing malaria over the course of one year which if. This approach protected all 9 clinical trial participants who received 3 high-dose vaccinations.
Listing a study does not mean it has been evaluated by. Such protection is thought to be mediated by CD8 T cells in the liver that secrete interferon-γ IFN-γ. The Way Forward The RTSSAS01 vaccine and the PfSPZ vaccine products are two of the most promising malaria vaccine candidates to date.
Results of Phase 3 testing show that among children aged 517 months who received 4 doses of RTSSAS01 vaccine efficacy against malaria was 36 over 4 years of follow-up. Mosquitoes carrying the deadly malaria parasite have flown along next to humans for thousands of years and the disease appears in documented reports as early as 2700 BC. Recipients take antimalarial medication chloroquine to prevent an actual malaria infection.
July 13 2021 Malaria vaccines provide strong and lasting immunity At a Glance Researchers developed a malaria vaccination strategy that provided broad long-lasting protection in controlled clinical trials. What is a Phase 3 clinical trial. Malaria and Malaria Vaccine Candidates.
Decades of endeavor have taught that achieving this goal will be challenging. Phase 3 trial results for vaccine RTSSAS01. Based on the malaria vaccine clinical trial experiences key ethical practices for effective clinical trial research in low-resource settings are described.
Research continues with other malaria vaccines. Phase III Trial of a Malaria Vaccine in Adults Living in the United States of America The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Pf sporozoites PfSPZ administered by mosquito bites are the only immunogens shown to induce such protection in humans.
In this double-blind randomised controlled phase 2b trial the low-dose circumsporozoite protein-based vaccine R21 with two different doses of adjuvant Matrix-M MM was given to children aged 517 months in Nanoro Burkina Fasoa highly seasonal malaria transmission setting. Vivax remains a key priority. While malaria vaccine development has made significant progress in recent years no trials of malaria vaccines have ever been conducted in pregnant women.
The RTSSAS01 malaria vaccine is being introduced sub-nationally in phased pilot introductions through the EPI programmes in Malawi Ghana and Kenya. Final results of a phase 3 individually randomised controlled trial The Lancet Vol 387 No. The most effective malaria vaccine is R21Matrix-M with a 77 efficacy rate shown in initial trials and significantly higher antibody levels than with the RTSS vaccine.
1 Malaria continues to plague humans today causing hundreds of thousands of deaths each year. The development of highly effective and durable vaccines against the human malaria parasites Plasmodium falciparum and P. Malaria Vaccination with or without Chemoprevention This trial assessed the efficacy of the vaccine RTSSAS01E as compared with chemoprevention in preventing malaria.
RTSSAS01 is the first malaria vaccine to be tested in Phase 3 clinical trials and the first to be assessed in routine immunization programs in malaria-endemic areas. The protective efficacy of RT. On February 7 2022 the UCSF Malaria Elimination Initiative MEI hosted a presentation and QA with Dr.
10016 p367375 23 January 2016. June 30 2021 Two US. Our goal is to develop a vaccine that sustainably prevents Plasmodium falciparum Pf malaria in 80 of recipients.
Each trial has utilized a unique combination of antigens delivery platforms and adjuvants which has provided the research com. If the approach proves successful there chemoprophylaxis vaccination or CVac potentially could help reverse the stalled decline of global malaria. However recent innovation in malaria vaccine research and a diverse pipeline of novel vaccine candidates for clinical.
Quote The Jenner Institute director Prof Adrian Hill said the malaria vaccine would be tested on 4800 children in Africa next year after early trials yielded promising results. It is the first vaccine that meets the World Health Organization s WHO goal of a malaria vaccine with at least 75 efficacy. In an interview with the Times Hill said malaria was a public health emergency.
Significant progress toward a malaria vaccine specifically for Plasmodium falciparum has been made in the past few years with the completion of numerous clinical trials. Efficacy and safety of RTSSAS01 malaria vaccine with or without a booster dose in infants and children in Africa. Currently there is no vaccine in widespread use for the mosquito-transmitted disease.
The cluster randomised study in africa is already under way in malawi ghana and kenya where 720 000 children will receive the rtss vaccine known as.

The History Of The Rts S As01 Malaria Vaccine Trial The Lancet
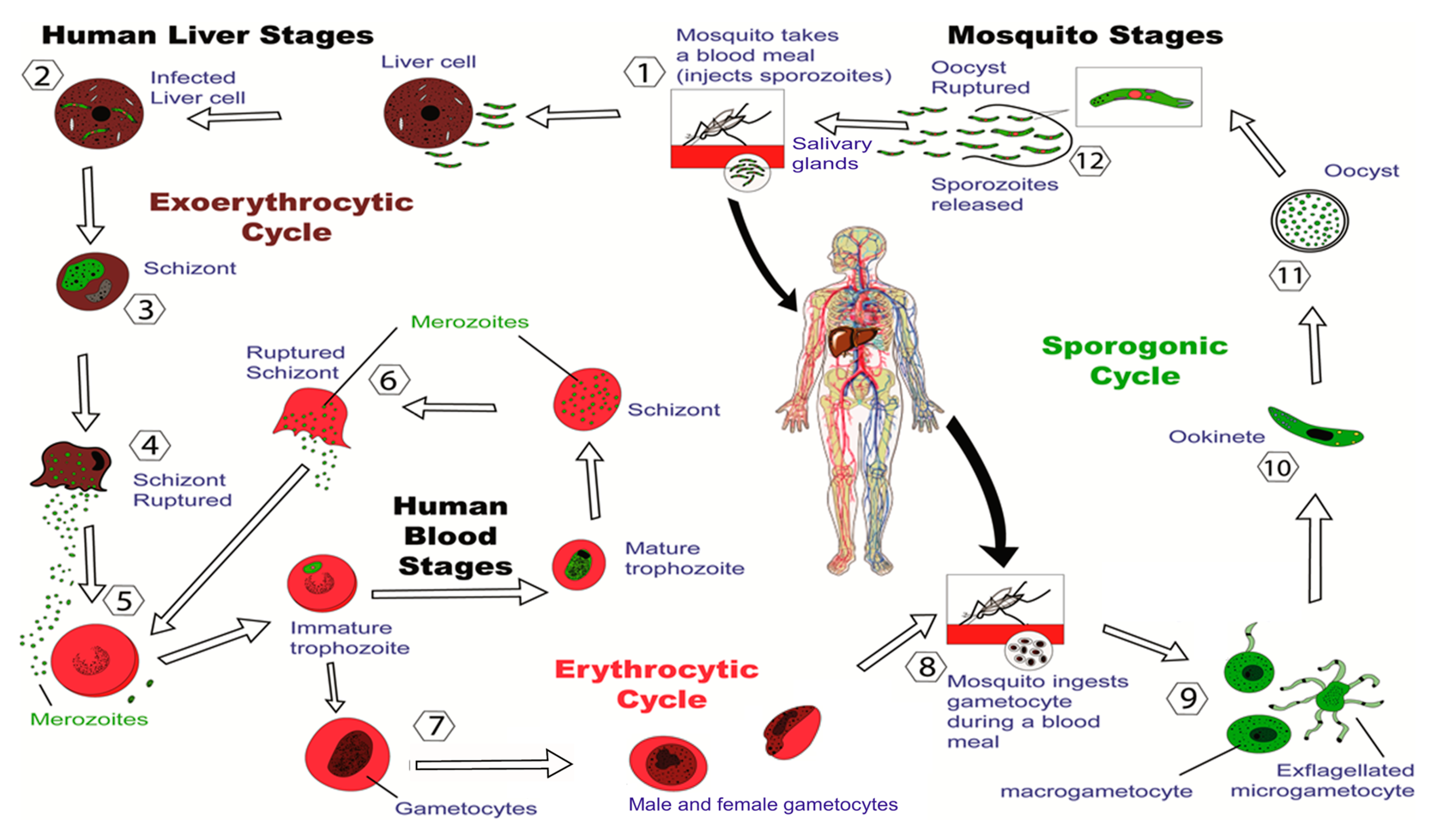
Vaccines Free Full Text Progress In The Development Of Subunit Vaccines Against Malaria Html

World S First Malaria Vaccine Gets Who Recommendation Goats And Soda Npr

Cryoport Developing Cold Chain Logistic Models For Malaria Vaccine

Seven Year Efficacy Of Rts S As01 Malaria Vaccine Among Young African Children Nejm

Current Malaria Vaccines Under Preclinical Development Or In Clinical Download Table
Schedule On The Malaria Vaccine Development For Approximately 50 Years Download Scientific Diagram
2

Efficacy Of A Low Dose Candidate Malaria Vaccine R21 In Adjuvant Matrix M With Seasonal Administration To Children In Burkina Faso A Randomised Controlled Trial The Lancet

World S First Malaria Vaccine Launched In A Pilot Program

Oxford Malaria Vaccine Proves Highly Effective In Burkina Faso Trial Malaria The Guardian

Malaria Vaccines Malaria Site
Malaria Vaccine World Health Organization Approve Malaria Vaccine For Africa Bbc News Pidgin

World S First Malaria Vaccine Given Go Ahead For Immunisation Of African Children Bbc Science Focus Magazine
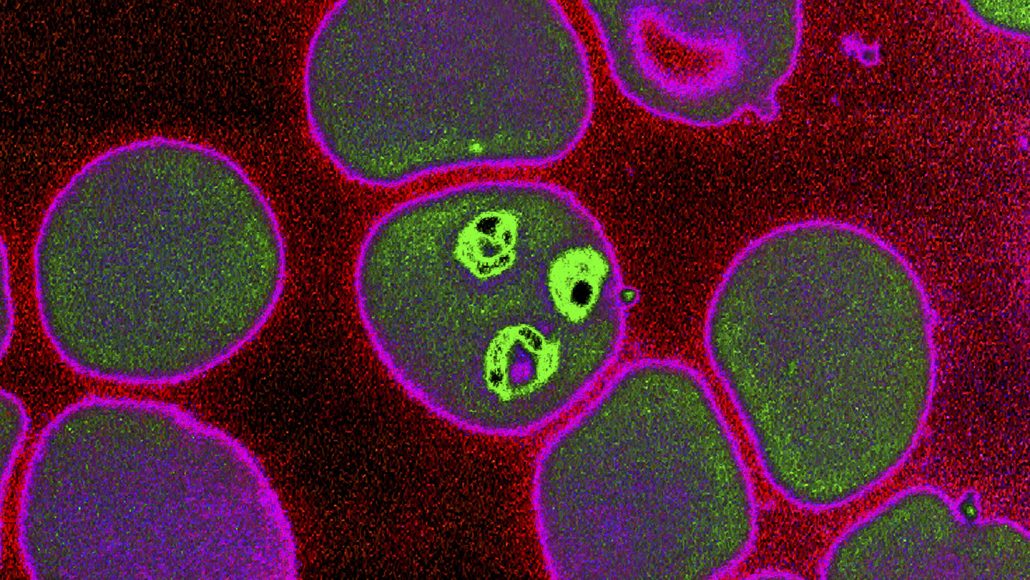
A Malaria Vaccine With Live Parasites Shows Promise In A Small Trial Science News